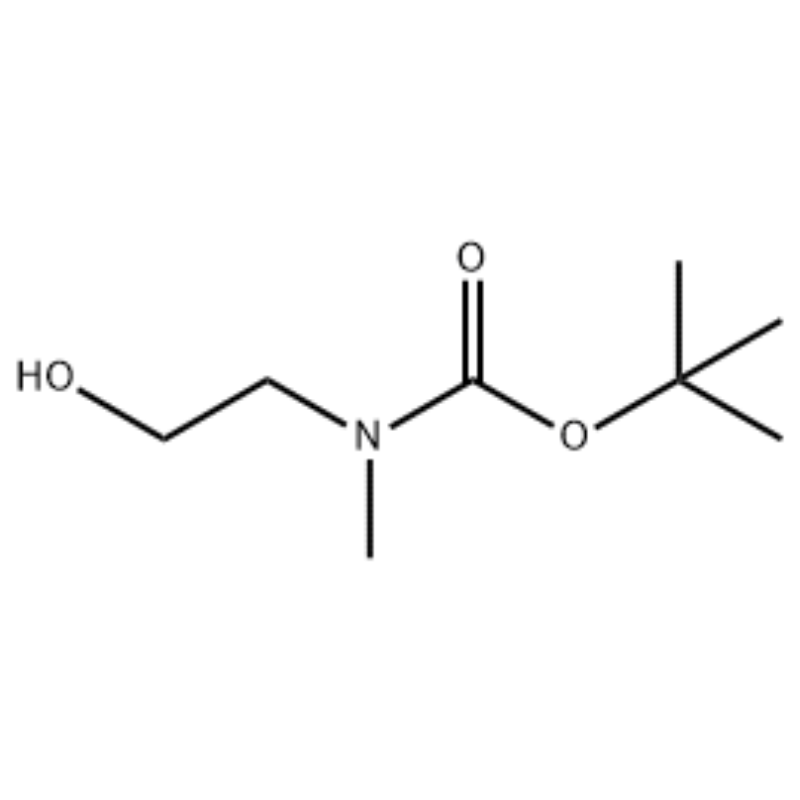
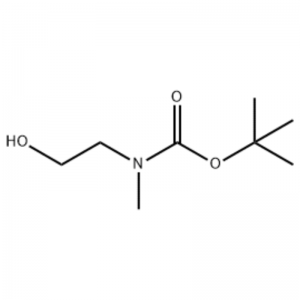
Ad solutionem 2-(methylamini) ethanoli (500 mg, 0.53 ml, 6.66 mmol) in CH2Cl2 (20 ml) addita Boc2O (1.48 g, 6.79 mmol), sequitur excitando ad cella temperiem pro 1 hora.Solutio reactionis extracta cum muria et CH2Cl2.Stratum organicum sic consecutum super MgSO4 et eliquata exsiccata est.Deinde liquamen in vacuo contractum erat ad obtinendum obiectum compositum (oleum sine colore, quantitatis);1H NMR (200 MHz, CDCl3) della 3.74 (q, J= 10.5, 5.2 Hz, 2H) 3.25 (t, J= 5.2 Hz, 2H) 2.91 (s, 3H) 1.45 (s, 9H);massa spectrum m/e (relativorum intensio) 144 (20) 102 (24) 57 (70) 44 (100).
Exemplum 38;N1-(3-Fluoro-4-(2-(1-(2-(methylamino)ethyl)-1H-imidazol-4-yl)thieno[3,2-b]pyridin-7-yloxy)phenyl)-N3 -(2-methoxyphenyl)malonamide (96);Gradus 1: tert-Butyl 2-hydroxyethyl(methyl)carbamatum (97) (J. Med. Chem., 1999, 42, 11, 2008) Ad solutionem 2-(methylamini) ethanoli (5.0 g, 67 mmol) in THF (50 ml) apud RT addita est Boc2O (15.7 g, 72 mmol) et reactionem mixturam in RT per 4 horas commota est.Reactio mixtionis ad siccitatem coacta et cum titulo compositi 97 adhibita est directe in proximo gradu cum nulla purificatione addito (11,74 g, 100% cedere).MS (m/z): 176.2 (M+H).
Praeparatio l-2-[4-bromo-2-(4-oxo-2-ftiotaioxo1hiazolidin-5-ylidenemefliyl)phenoxy]efliyl-3-efliyl-l- methylurea(Compoiotamd 161) Step 1 : Synthesis of t-butyl2- hydroxyethylmethylcarbamate;Ad solutionem 2-(methylamini)ethanol (500 mg, 0,53 ml, 6.66 mmol) in CH2Cl2 (20 ml) addita BoC2O (1.48 g,6.79 mmol), sequitur excitando ad cella temperiem pro 1 hora.Solutio reactionis extracta cum muria et CH2Cl2.Stratum organicum sic consecutum super MgSO4 et eliquata exsiccata est.Deinde liquamen in vacuo ad obtinendum compositum (oleum sine colore quantitatis); 1HNMR (200 MHz, CDCl3) della 3.74 (q, J= 10.5, 5.2 Hz, 2H) 3.25 (t, J= 5.2 Hz; 2.91 (s, 3H) 1.45 (s, 9H);massa spectrum m/e (relativorum intensio) 144 (20) 102 (24) 57 (70) 44 (100).
2-(methylamino)ethanolum (90.1 g, 1.2 mol) dissolutum est in 1.2 L chloridi methyleni, et BoC2O (218 g, 1 mol) lente adiectum est dum movens 00C, sequitur ad cella temperiem per 3 horas.Mixtura continue lota est cum 700 mL solutionis aqueae chloridi ammonii saturati, et aquae 300 mL.Mixtura lota dehydrata utens sodium anhydroum sulfate et coacto sub pressione reducto, ad obtinendum compositum (a) (175 g, 1 mol, 100) ut oleum nullo colore. TLC : Rf = 0.5 (50% EtOAc in Hex) subjicitur cum Ce-Mo stain1H NMR (600MHz, CDCl3) della 1.47 (s, 9H), 2.88 (s, IH), 3.41 (s, 2H), 3.76 (s, 2H).
90,1 g (1.2 mol) 2-(methylamino) ethanoli dissolutum est in 1.2 L chloridi methyleni, 218 g (1 mol) Boc2O lente additur dum solutio consequens in 0C commota est, et solutio inde commota est. locus temperatus pro 3 horis.Reactio mixtura sequentiter abluta est cum 700 mL solutionis aquei saturatae Ammoni chloridi et 300 mL aquae, dehydratae utens sulphate sodium anhydroum, et deinde coactum sub pressionem reductam ad obtinendum 175 g (I mol) olei achromici compositi ab eo muniti. Boc coetus (cede: 100%).[0140] 1H NMR (600MHz, CDCl3) della 7.84 (br s, 2H), 7.76 (d, J = 15.0 Hz, 2H), 3.63 (d. , J = 15.0 Hz, 3H), 1.46 (d, J = 16.2 Hz, 9H) [0141A] 90 g (0.514 mol) compositi in 1.5 L tetrahydrofuran, 88.0 g (539 mol) resoluto. hydroxyphthalimide et 141 g (0.539 mol) triphenylphosphini adiectae, 106 mL (0.539 mol) diisopropyl azodicarboxylatis lente adiectae sunt, solutionem inde in 0C moventem, et solutio inde per 3 horas excitata est dum temperatura eius levata est. ad locus temperatus.Post intentionem reactionis mixtionis sub pressionibus reductis, DC mL isopropyletheris adiecta est, solutio consequens ad 0C horam 1 commota est, et oxydatum solidi album triphenylphosphinum eliquatum est.Solidum cum 200 mL isopropyletherarum lavatum ad 0C refrigeratum et cum filtratu primo coactum est, et inde filtratum coactum sub pressione reducta ad obtinendum 198 g mixtionis Compositi XX et diisopropyl hydrazodicarboxylati in mixtione ratione 10 ad 15%. (cede: 120%).[0142] 1H NMR (600MHz, CDCl3) della 7.84 (br s, 2H), 7.76 (d, J = 15.0 Hz, 2H), 3.63 (d. , J = 15.0 Hz, 3H), 1.46 (d, J= 16.2 Hz, 9H).
 Aedificium XII, No.309, Meridionalis 2nd Via, Economic Zona, Longquanyi Districtus, Chengdu, Sichuan, China.
Aedificium XII, No.309, Meridionalis 2nd Via, Economic Zona, Longquanyi Districtus, Chengdu, Sichuan, China. amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com
amy@enlaibio.com / cynthia@enlaibio.com / edison@enlaibio.com / daisy@enlaibio.com +86 (028) 84841969
+86 (028) 84841969 +86 135 5885 5404
+86 135 5885 5404



















.png)


